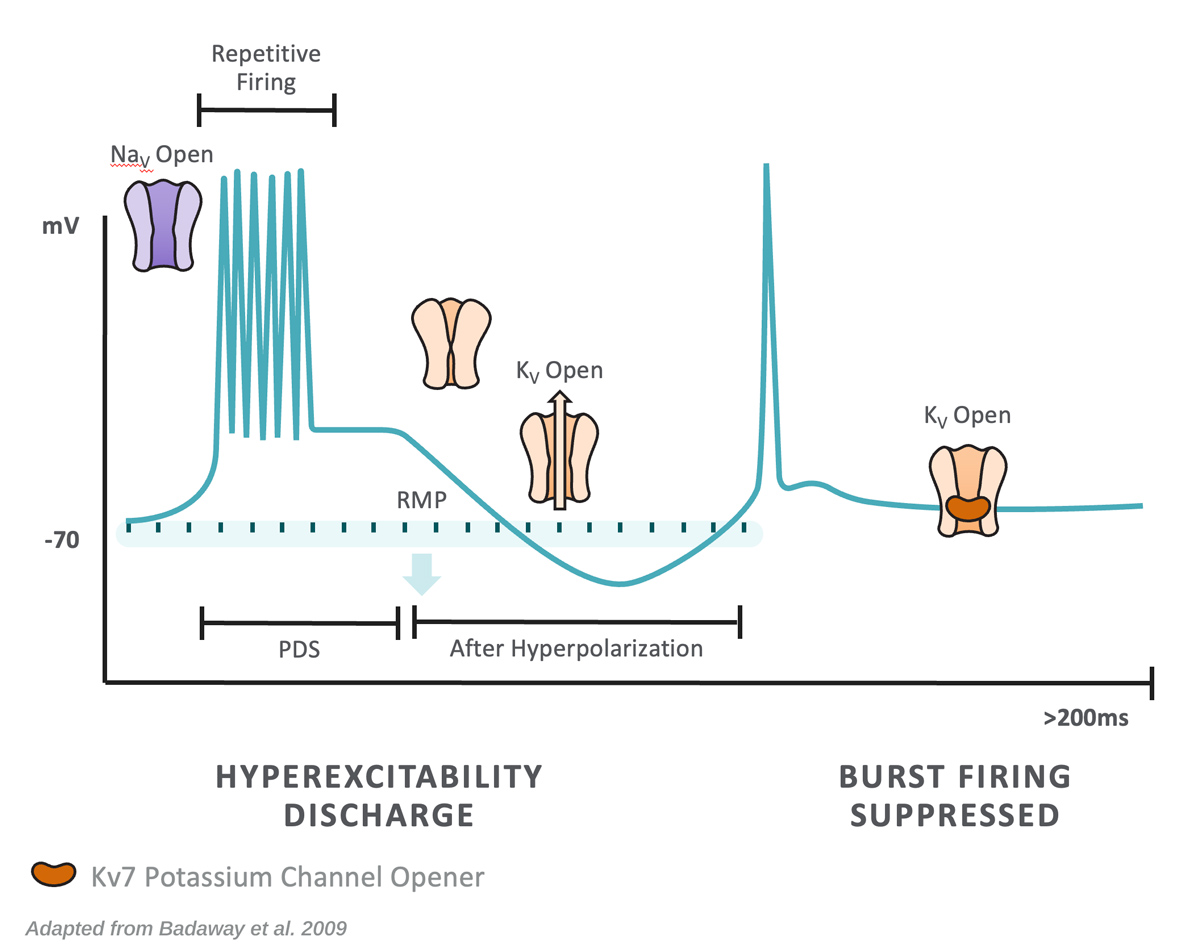
Phase 3 Primary Generalized Tonic-Clonic Seizures: X-ACKT
Study Design3
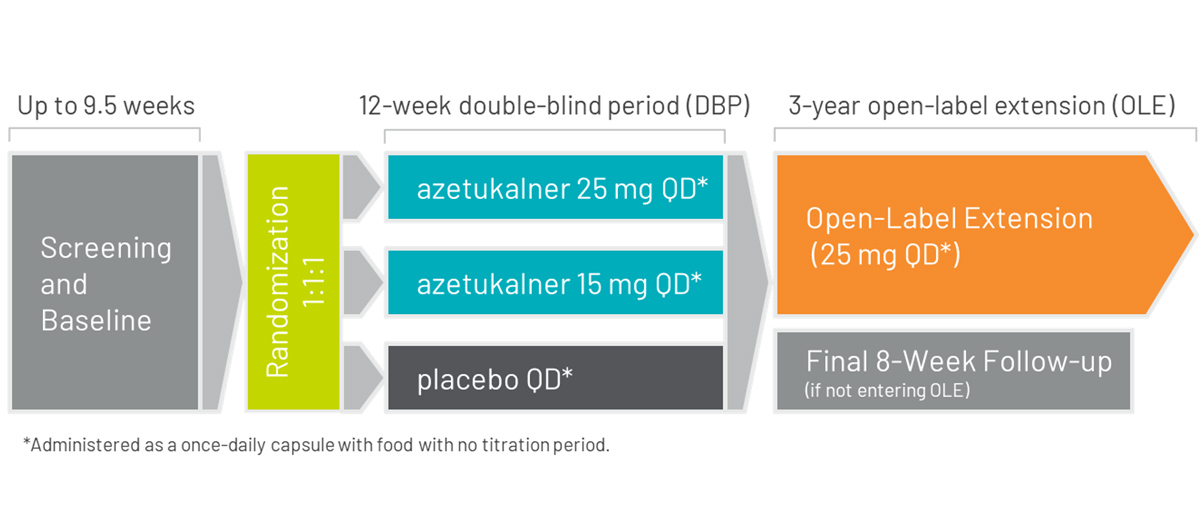
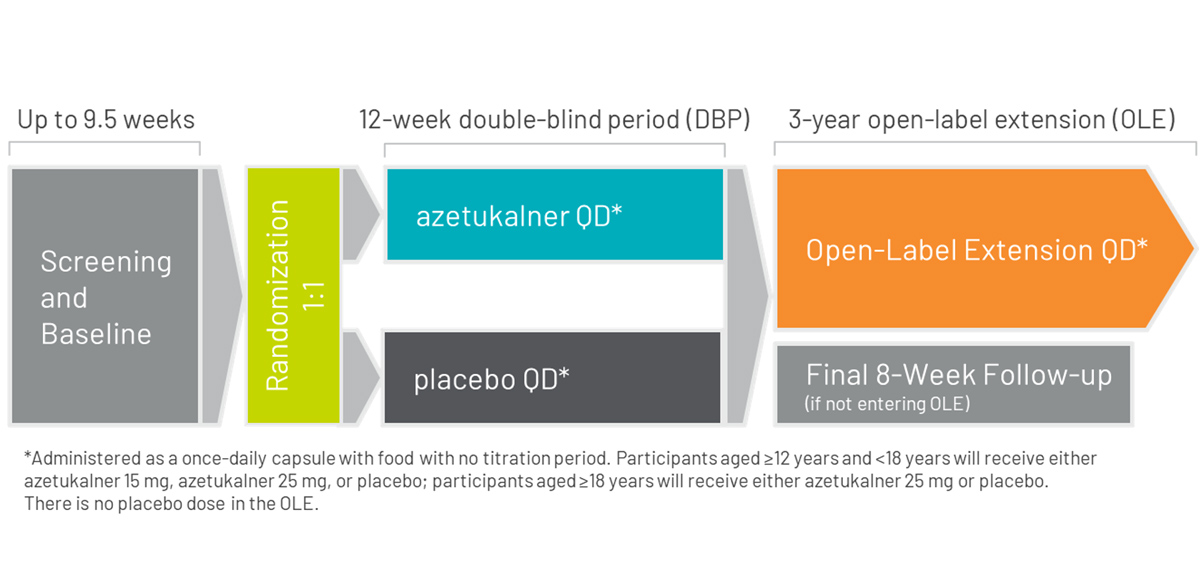
X-ACKT is a Phase 3, multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy, safety, and tolerability of azetukalner as adjunctive treatment in adults aged ≥12 years with a seizure frequency of ≥3 PGTCS during the last 8 weeks of the baseline period and taking 1 to 3 ASMs.
Approximately 160 eligible subjects will be randomly assigned 1:1 (azetukalner 25 mg : placebo taken QD with food).
ASM, antiseizure medication; PGTCS, primary generalized tonic-clonic seizures.
Screening/baseline period: Up to 9.5 weeks duration to assess the frequency of seizures.
Double-blind period (DBP): 12 weeks duration. There is no titration period.
Follow-up period: 8 weeks duration after the last dose of study drug for subjects who do not complete the 12-week DBP or who complete the DBP but do not enter the OLE study.
Azetukalner is administered as a once-daily capsule with food with no titration required. Subjects aged ≥12 years and <18 years will receive either azetukalner 15 mg, azetukalner 25 mg, or placebo; subjects aged ≥18 years will receive either azetukalner 25 mg or placebo.3
On completion of the double-blind period in X-ACKT, eligible patients may enter an open-label extension study for up to three years.13